Additionally, there are challenges that must be addressed to improve clinical trials. There is a vital need for biomarkers in progressive MS that can measure progression and indicate if a potential treatment is effective. Clinical trials also need to be designed that can provide deeper insights into the biology of progression which will lead to targeting areas where a drug intervention could be successful in preventing disease progression or stimulating nervous system repair. Finally, clinical trials need to include a broader spectrum of people with progressive MS and people affected by MS need to be involved in the design of trials.
The Alliance is working in multiple ways to speed up and improve clinical trials:
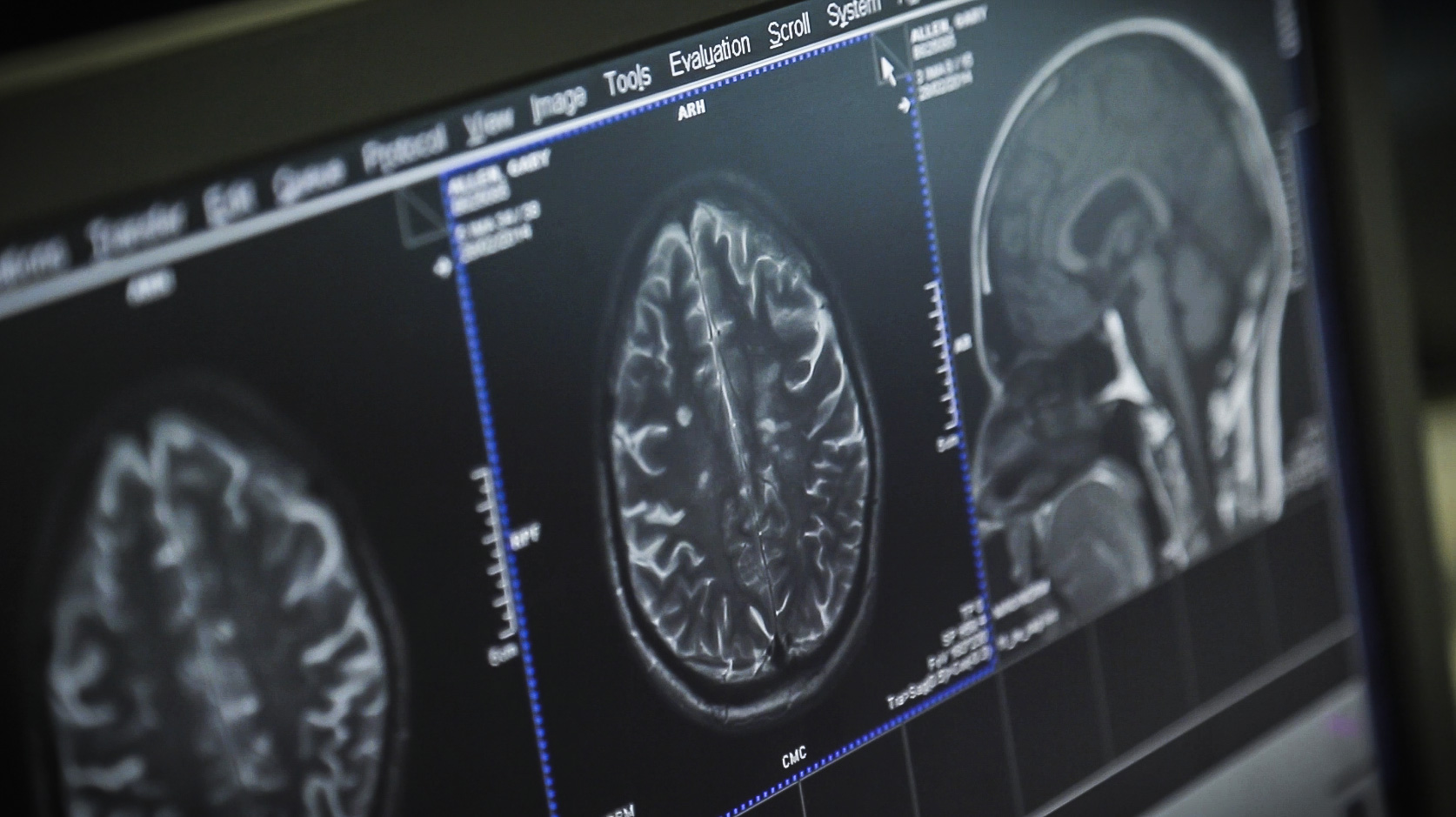
- MRI Imaging Biomarker International Collaborative Research Network – Artificial Intelligence can lead to new tools that can predict, via MRI’s, the changes in the brain as MS progresses. Such tools could be used to quickly evaluate whether a potential treatment is effective and ultimately be used in a clinical setting for customizing an individual’s treatment. The Alliance has invested €4 million in a research network that is using machine learning to create tools that would be a major advancement in progressive MS clinical trials.
- Serum Neurofilament light chain (sNfL) – a fluid biomarker – If sNfL is further confirmed as a biomarker for progressive MS, a blood test could help predict the future course of the disease and indicate whether a treatment is working. The Alliance has formed an expert panel that has published a set of recommendations and is working with the FDA, EMA and the MS scientific community to further this effort.
- Expert panel recommendations – the Alliance has convened a panel of experts to give recommendations on ways to improve progressive MS clinical trials. This panel has produced a paper that provides a number of steps for the design of trials in progressive MS that will not only effectively test new treatments but also lead to new understandings of the biology of progression. The panel also describes the importance of including different cohorts of people with progressive MS in trials and that people affected by MS should be involved in trial design. Next steps in this work include Alliance funding initiatives that would support the testing of new clinical trial approaches.